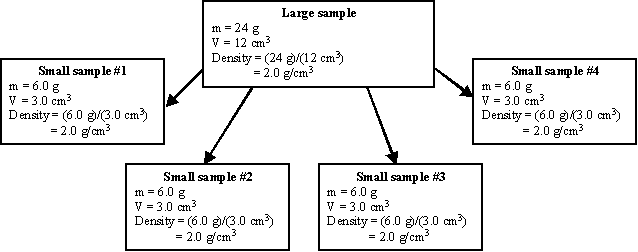Competency #3
Answer and Explanation
The correct answer is A.
![]()
Explanation without numbers: The density of the substance does not change with the amount of sample present. Therefore, the density of each new sample is 2.0 g/cm3. Density, like temperature, is an intensive property . For example, a person that floats in a small pool will also float in a pool the size of a football stadium. This is because the density of pure water does not change based on how much of it you have (note: sea water does have a different density than fresh water- this is why ‘pure' water was specified above).
Explanation with numbers: In this example, the original sample had a density of 2.0 g/cm3. The size of the original sample is not given, but we will use a 24 g sample to illustrate how the density is able to remain the same. The volume of a 24 g sample with a density of 2.0 g/cm3 is 12 cm3. If this sample is broken into 4 equal pieces, each piece will have a mass of 6.0 g. Additionally, each sample will have a volume of 3.0 cm3. The density of each new sample is: Density = (6.0 g)/(3.0 cm3) = 2.0 g/cm3, the same as before!