Competency #3
Answer and Explanation
![]()
The density of water is 1.0 g/mL. Objects will float in water when they are able to displace enough water to support their weight, which happens when the density of the object is less than the density of water.
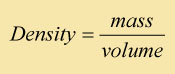
So, if diet sodas float and regular sodas do not, diet sodas must be less dense than regular sodas.
Why might this be? Well, the volume of soda is the same in 12 oz cans, so the volume isn't changing. So it must be that the mass of diet sodas is less than the mass of regular sodas.
Why would this be? They both have water, and carbonic acid, and colorings and flavorings… but regular soda has sugar while diet sodas are sweetened with aspartame.
Both sugar and Aspartame increase the density of the soda. However, it turns out that regular sodas have 39 grams of sugar (this is about 12 teaspoons!), but only about 0.1 g of Aspartame, (because it is a much more intense sweetener). As a result, there is more mass in a regular soda, so it is more dense than diet soda.
Based on this experiment, we can also conclude that diet sodas are less dense than water, while regular sodas are more dense than water.