Competency #5
Answer and Explanation
![]()
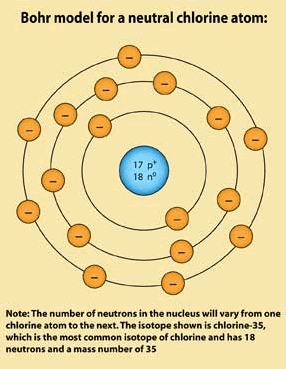
Read through the following description to see if your Bohr model for chlorine matches the one described:
To draw a Bohr model, we look at the periodic table and see that the atomic number for Chlorine is 17 (this is found directly above the symbol 'Cl' in the next to last column of the period table). The atomic number will always be equal to the number of protons an atom has, so we indicate that there are 17 protons in the nucleus by writing '17 p+' in a small circle representing our nucleus at the center of the atom.
Since the Chlorine atom was specified in the problem to be neutral, we know there must be 17 electrons to offset the 17 protons. Then, we recall that the 1st electron shell can hold 2 electrons, while the 2nd and 3rd shells can each hold 8 electrons. We draw a circle around the nucleus to represent the 1st shell.
We then use 2 of the 17 electrons to fill the first shell. Since the 1st shell is full, we draw a 2nd shell, and use 8 more of our electrons to fill it. We used 2 electrons in the 1st shell and 8 more in the 2nd shell for a total of 10 used out of 17. We have 7 left that we can put in our 3rd shell.
Now we can clearly see that there are 7 electrons in the outer shell of a neutral Chlorine atom. Compare your diagram to the completed Bohr model for chlorine.