Competency #6
Answer and Explanation
![]()
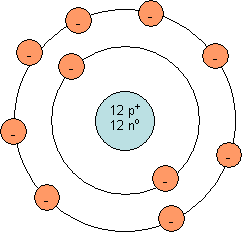
Bohr model for a neutral magnesium atom:
Note: The number of neutrons in the nucleus will vary from one magnesium atom to the next. The isotope shown is magnesium-24, which is the most common isotope of magnesium and has 12 neutrons and a mass number of 24.
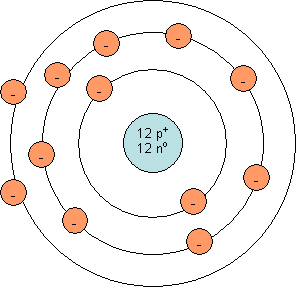
As you can see, a neutral magnesium ion does not have a full outer shell. The outer shell has 2 electrons, but a capacity for 8 electrons. It is quite easy for magnesium to lose two electrons. Once this happens, the outermost occupied shell is full (see diagram below). This ion contains 12 protons and 10 electrons (2 less than the neutral atom). Since it contains 12 positively charged protons and 10 negatively charged electrons, it has a charge of +2. This is the reason magnesium tends to form Mg2+.
Bohr model for a magnesium atom: