Competency #6
Answer and Explanation
![]()
Draw a Bohr model for a Cl2 molecule.
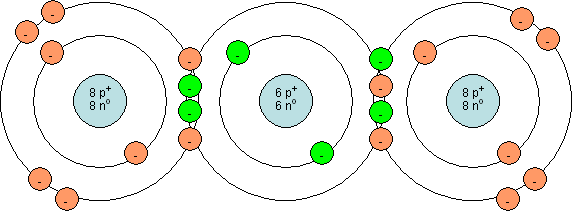
Each of the two oxygen atoms have 8 protons and 8 electrons. The carbon atom has 6 protons and 6 electrons. The carbon and the oxygen atom on the left share 4 electrons, while the carbon atom also shares 4 electrons with the oxygen atom on the right. Now, we see that the outer electron shell of each atom is full (has 8 electrons, which is the maximum for the 2nd shell).
What type of bond is formed between the carbon and oxygen atoms?
Two double covalent bonds are formed in CO2.
What is the overall charge on the CO2 molecule?
The overall charge on any molecule is zero. However, we can show this for the above molecule by adding up the number of protons and electrons. There are 6 protons in the nucleus of carbon and 8 in the nucleus of each oxygen, for a total of 22. Now, we count all of the electrons and see that there are 22 electrons. Since there are equal numbers of protons and electrons, the molecule has a zero overall charge.