Competency #7
Answer and Explanation
![]()
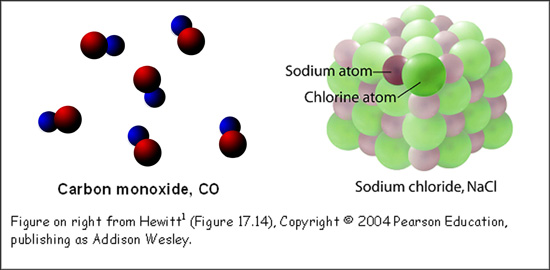
The formula for a molecular compound tells us the specific number of atoms in a single molecule. For example, a CO molecule contains 1 carbon atom and 1 oxygen atom. Multiple CO molecules can exist in close proximity to one another, but each one is separate and able to move about on its own (see figure, below left).
The formula for an ionic compound tells us the ratio of one type of atom to another. For example, NaCl contains 1 sodium atom for every 1 chlorine atom. NaCl is more commonly known as table salt. A single granule of table salt contains millions of sodium atoms and millions of chlorine atoms, in a 1:1 ratio. Shown in the figure (below right) is a representative sample of NaCl.
© 2008 Anne Arundel Community College