Competency #7
Answer and Explanation
Explain the difference between the following:
NaCl(s) and NaCl (aq)
![]()
NaCl(s) refers to salt in the form of a solid, like the salt in your salt shaker. NaCl(aq) refers to table salt that has been dissolved in water, hence it is aqueous.
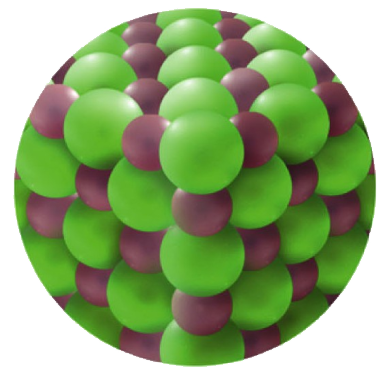
Sodium chloride is an ionic compound, with a Na+1 ion and a Cl-1. In the solid form, these ions are arranged in an alternating manner (in a 3-d structure). See picture below left.
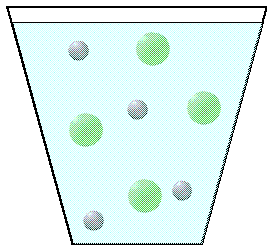
Ionic compounds that are soluble separate into ions when placed in water. The picture of four NaCl molecules dissolved in water are shown below right.
 |
 |
| NaCl(s) Picture from Hewitt, et al1 |
NaCl(aq) |
![]()
Hewitt, Paul G., John Suchocki, Leslie A. Hewitt, Conceptual Physical Science, 3rd Edition, San Francisco: Addison Wesley, 2004.
© 2008 Anne Arundel Community College