Competency #11 & #5
Answer and Explanation
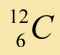 |
98.9% |
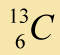 |
1.1% |
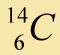 |
0.0000000001% |
Belief: Carbon is a common element, found in plants, animals, rocks, and many other places. There are 3 isotopes that occur naturally. A sample of carbon atoms is analyzed using a mass spectrograph. This experiment (and many other similar experiments) shows the following relative abundance for each isotope of carbon:
Given the above belief, which of the following is a believable statement?
![]()
Answer:
The number of protons is equal to the number of neutrons for carbon, but not other elements. While this is often true for carbon, it is NOT true about 1.1% of the time.
The number of protons is equal to the number of neutrons in certain cases. This is correct. For the first 20 elements on the periodic table, this is often the case. However, it is not always true for any element. For example, it is true for carbon 98.9% of the time. It is never true for fluorine, which is found to exist with 9 protons and 10 neutrons 100% of the time.
The number of protons is always equal to zero. This answer does not make sense. There are no atoms that have zero protons.
The number of neutrons is always equal to the number of electrons. The number of neutrons is equal to the number of electrons, but there are many instances where this is not true. For example, a neutral carbon-13 atom has 6 protons, 7 neutrons, and 6 electrons.