Competency #6
Predict chemical bonding in terms of electron behavior and the periodic table
Learning Objectives e & f
- e. Explain ionic bonding using the Bohr (electron shell) model
- f. Predict the ionic compound that will form from two elements
![]()
Question
Write down your answer to the following question:
Shown below are Bohr models for magnesium (left) and chlorine (right):
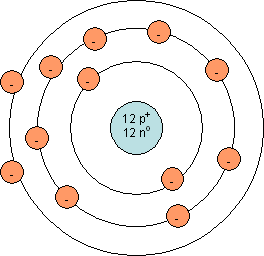
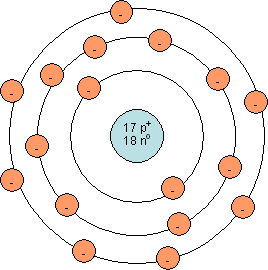
Which of the following statements best describes the ionic bond between magnesium and chlorine?
- One magnesium ion will form an ionic bond with one chlorine ion
- One magnesium ion will form an ionic bond with two chlorine ions
- Two magnesium ions will form ionic bonds with one chlorine ion
- The outer electron shells of magnesium and chlorine will overlap
- No ionic bonds can form between magnesium and chlorine
© 2008 Anne Arundel Community College
