Competency #5
Analyze atomic structure in terms of the Bohr model.
Learning Objectives b & c
- b. Calculate the number of neutrons for the most common isotope of an element using the periodic table.
- c. Explain how one isotope differs from another.
Competency #11
Identify the processes involved in learning
Learning Objective a
- a. Identify current understandings and/or long-held beliefs that are incorrect
![]()
Question
Write down your answer to the following question:
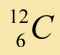 |
98.9% |
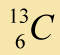 |
1.1% |
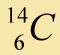 |
0.0000000001% |
Belief: Carbon is a common element, found in plants, animals, rocks, and many other places. There are 3 isotopes that occur naturally. A sample of carbon atoms is analyzed using a mass spectrograph. This experiment (and many other similar experiments) shows the following relative abundance for each isotope of carbon:
Given the above belief, which of the following is a believable statement?
- The number of protons is equal to the number of neutrons for carbon, but not other elements
- The number of protons is equal to the number of neutrons in certain cases
- The number of protons is always equal to zero
- The number of neutrons is always equal to the number of electrons
© 2008 Anne Arundel Community College
