Competency #3
Answer and Explanation
![]()
A. By pumping some of the air molecules out of the container, the pressure felt by the banana will decrease . In this case, the temperature is unaffected, so each molecule is colliding with the walls and the banana with the same frequency and force as before, however there are fewer air molecules present. This means that the banana and the walls of the container feel less collisions in a given time period than they did before. Fewer collisions at the same level of force will exert a lower pressure.

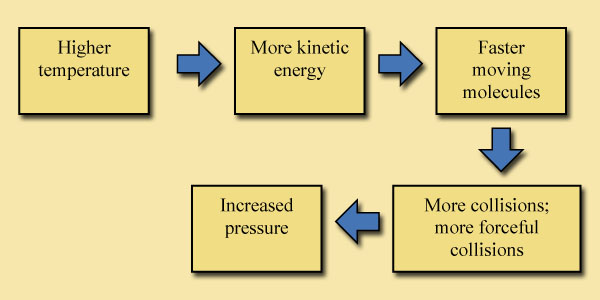
B. By increasing the temperature in the container, the pressure felt by the banana will increase . In this case, the number of air molecules inside the container remains the same, while the temperature goes up. The air temperature is a measure of the average kinetic energy of the air molecules in the container. A temperature rise indicates that each air molecule, on average, has more kinetic energy than it did before. Each air molecule is going faster than it was before, and is, therefore, colliding with the walls more often and with more force than before. Remember, it is the sum of all these tiny collisions with objects that makes up pressure. Collisions that are more forceful and greater in number will therefore cause an increased pressure on the walls of the container and on the banana.