Competency #3
Answer and Explanation
The correct answer is B.
![]()
At the top of Mt. Everest, there is less atmospheric pressure than at sea level. This is because you are closer to the top of the atmosphere (just like as you go deeper in water, the pressure increases)
Top of atmosphere
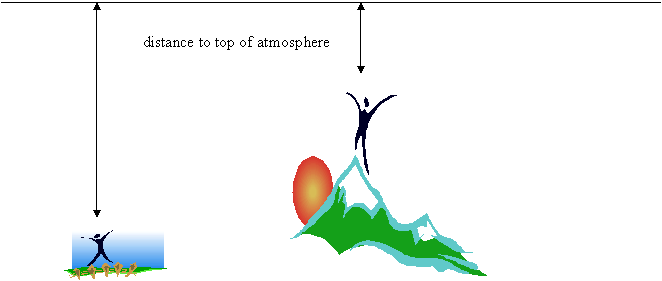
If the air pressure is decreased, there is less force that the water molecules have to act against to escape the beaker and become a gas. This means it requires less kinetic energy for water to leave the liquid phase and become a gas.
Since temperature is a measure of kinetic energy, if the water needs less kinetic energy to become a gas, the water will become a gas at a lower temperature.
© 2008 Anne Arundel Community College