Competency #5
Answer and Explanation

6 - The number above the element symbol is called the atomic number, and represents the number of protons in an atom of that element. That means that any atom of carbon will have 6 protons. (All isotopes and ions of carbon have 6 protons. If a particle has 6 protons it is some form of carbon).
12.01 - The number below the element is called the mass number, and represents the average atomic mass. The atomic mass is the number of protons plus the number of neutrons. Three different isotopes for carbon occur naturally, 12C, 13C , 14C . Approximately 99% of all carbon atoms are carbon-12, 1% carbon-13, and a trace amount of C-14. The average mass is a weighted average, as shown here:
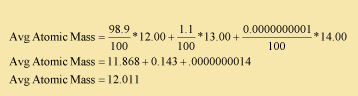
© 2008 Anne Arundel Community College