Competency #5
Answer and Explanation
The correct answer is 14 neutrons.
![]()
As stated in the question, we can usually round the average atomic mass to get the mass number for the most common isotope. From the periodic table, we see that the average atomic mass for aluminum is 26.98. Rounding this, we get a mass number of 27. Thus, we assume the most common isotope for aluminum is:
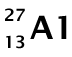
The atomic number for aluminum is 13 (also from the periodic table). The mass number is the sum of the number of protons and the number of neutrons. We can calculate the number of neutrons:
# neutrons = mass number – atomic mass
# neutrons = mass number – # protons
# neutrons = 27 - 13
# neutrons = 14
© 2008 Anne Arundel Community College