Competency #6
Answer and Explanation
![]()
Atoms in a group tend to react similarly because they have same number of valence electrons.
For example, compare the Bohr models for sodium (Na) and potassium (K) below:
| Bohr model for sodium (Na) | Bohr model for potassium (K) |
 |
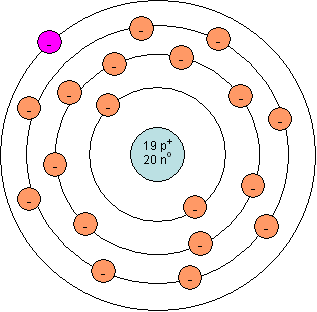 |
| Notice one valence electron (pink) | Notice one valence electron (pink) |
Because both of these atoms have one valence electron, they will lose this electron to complete the valence shell, becoming a +1 ion. Since both will form +1 ions they will react in the same ratio with other ions. For example, Na reacts with chlorine to become NaCl and K reacts with chlorine to become KCl. They both form compounds with oxygen in a 2 to one ratio, Na2O and K2O.
© 2008 Anne Arundel Community College