Competency #6
Answer and Explanation
![]()
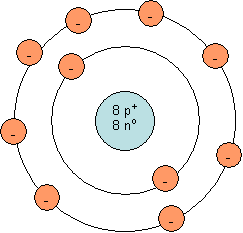
Bohr model for a neutral oxygen atom:
Note: The number of neutrons in the nucleus will vary from one oxygen atom to the next. The isotope shown is oxygen-16, which is the most common isotope of oxygen and has 8 neutrons and a mass number of 16.
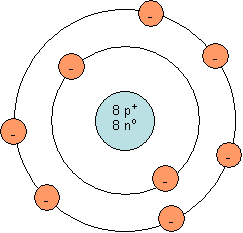
As you can see, a neutral oxygen ion does not have a full outer shell. The outer shell has 6 electrons, but a capacity for 8 electrons. It is easier for oxygen to gain two electrons than to lose 6 electrons. Once oxygen gains 2 electrons, the outermost occupied shell is full (see diagram below). This ion contains 8 protons and 10 electrons (2 more than the neutral atom). Since it contains 8 positively charged protons and 10 negatively charged electrons, it has a charge of –2. This is the reason oxygen tends to form O2-.
Bohr model for a neutral oxygen atom: