Competency #6
Answer and Explanation
![]()
Draw a Bohr model for a Cl2 molecule.
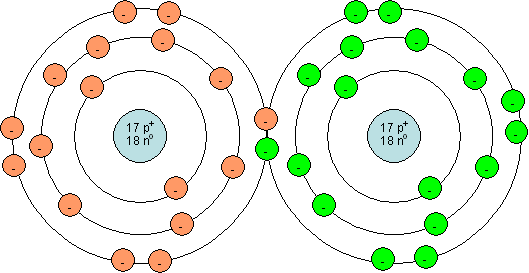
Two Cl atoms are shown above. Each atom has 17 protons and 17 electrons. By sharing two electrons, the outer electron shell of each atom is full (has 8 electrons).
What type of bond is formed between the two Cl atoms?
The type of bonding exhibited by the two chlorine atoms is a single covalent bond.
© 2008 Anne Arundel Community College