Competency #6
Answer and Explanation
The correct answer is C.
![]()
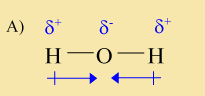
The above molecule contains two polar bonds. However, it is not a polar molecule because of the symmetry of the molecule. Another way to say this is that the molecule does not have both a positive and a negative end. Instead it has two positive ends and is negative in the middle.
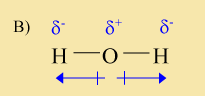
The above molecule contains two polar bonds. However, it is not a polar molecule because of the symmetry of the molecule. Another way to say this is that the molecule does not have both a positive and a negative end. Instead it has two negative ends and is positive in the middle.
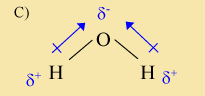
This is the correct answer. Unlike the molecules in choices A and B, this molecule is polar. It contains both a positive and a negative end. The top of the molecule is negative, while the lower parts are positive. Next, we check to see if it agrees with Table 19.19. This table shows electronegativities to be 3.44 for oxygen and 2.2 for hydrogen. Thus, oxygen is more electronegative and will pull more strongly on the electrons than hydrogen. This is in agreement with the above molecule, which shows the electrons being pulled toward oxygen, forming a partial negative charge on the oxygen and partial positive charges on the hydrogen atoms.
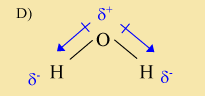
This molecule is polar. It contains both a positive and a negative end. The top of the molecule is positive, while the lower parts are negative. However, this diagram is in conflict with the electronegativity information from the table. As described in the answer C explanation, the oxygen is more electronegative, which means the electron will pull the electrons more strongly than the hydrogen atoms. In the above molecule, the opposite is shown- the hydrogen atoms are pulling on the electrons more strongly than the oxygen atom. Therefore, this cannot be the correct diagram for a water molecule.