Competency #7
Answer and Explanation
![]()
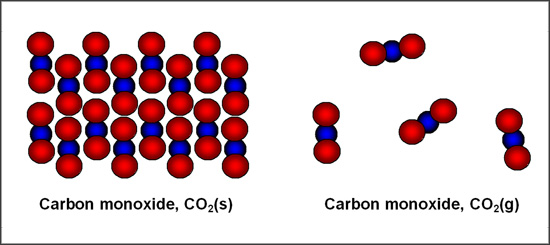
CO2(s) refers to solid carbon dioxide, while CO2(g) refers to gaseous carbon dioxide. However, both notations refer to a molecule containing 1 carbon atom and 2 oxygen atoms.
Shown below (left) are several carbon dioxide molecules that are in the solid phase. They are each fixed in their position. Shown below (right) are several carbon dioxide molecules in the gaseous phase. Each molecule is free to move around, and there is much more space between each molecule.
Solid carbon dioxide is more commonly known as ‘dry ice.' Gaseous carbon dioxide is produced in the combustion of any hydrocarbon, such as gasoline.
© 2008 Anne Arundel Community College