Competency #7
Answer and Explanation
N2(g) + 3 H2(g)![]() 2 NH3(g)
2 NH3(g)
![]()
Lets look at how this was obtained:
First lets count the number of each type of atom in the products and in the reactant to see what needs to be balanced.
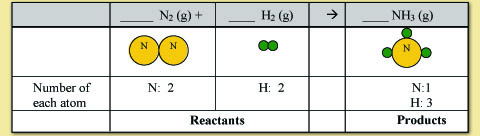
Now, check to see what you need to do to get the numbers the same for all of the elements on both side of the equation:
For this example, we will start with balancing nitrogen. It doesn't matter which element you start with, the final answer will be the same. Counting the nitrogen shows that you have 2 N atoms on the reactant side and 1 N atom on the product side. To balance this we need to put a 2 in front of NH3. Notice how that changes the numbers for nitrogen and hydrogen.
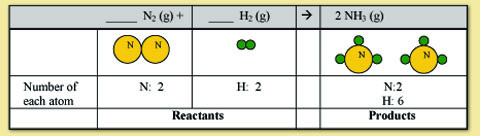
Nitrogen is now balanced but hydrogen is not. To balance hydrogen we need to get the 2 in the reactants to be the same number as the 6 in the products. We can do this by putting a 3 in front of the H2
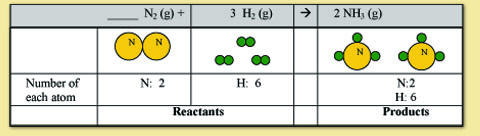
Note: The coefficient in front of the N2 is 1. When the coefficient is 1, chemists leave the coefficient blank. Thus, if there is no coefficient written, assume it is one. Now, all the atoms are balanced!