Competency #8
Answer and Explanation
Based on Coulomb's Law, rank the following sets of particles from greatest electrical repulsive force to least electrical repulsive force. Note: p + represents a proton and e - represents an electron.
The correct answer is: C > B > D.
![]()
Coulomb's Law is:
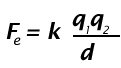
In this problem, there are two types of charges. The protons and electrons have nominal charges of +1 and –1, respectively. An attractive force will be felt between any two unlike charges. Therefore, only B, C, and D will experience an attractive force.
The electrical force is directly proportional to the size of the charges. That is, the bigger the electrical charges, the bigger the electric force.
The electric force is inversely proportional to the distance squared. That is, the farther apart the particles, the lower the electric force.
B and C have particles that are equally far apart. However, the charges are bigger in C, so this set of charges will have the largest electric force.
The particles in D will have a smaller electric force than those in B both because the particles in D have less charge AND are farther apart than those in B.