Competency #8
Answer and Explanation
![]()
In chemistry, the strength of ionic bonds is determined by the strength of the columbic attraction between the positive and negative ions. Use Coulomb's law to predict which of the following ionic compounds would have the strongest bond.
A. NaCl (Na+1, Cl-1)
B. CaS (Ca+2, S-2)
C. AlN (Al+3, N-3)
D. All would have the same bond strength
Answer:
The correct answer is C.
Coulomb's Law is:
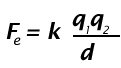
The electrical force is directly proportional to the size of the charges. That is, the bigger the electrical charges, the bigger the electric force.
Since ScN has the greatest charges, it has the greatest electrical force. This is reflected in a quantity called lattice energy. The lattice energies for these compounds are given below:
NaCl – 788 kJ/mol
CaO – 3414 kJ/mol
ScN – 7547 kJ/mol
Data taken from http://chemistry.umeche.maine.edu/~amar/fall2004/Lattice.html