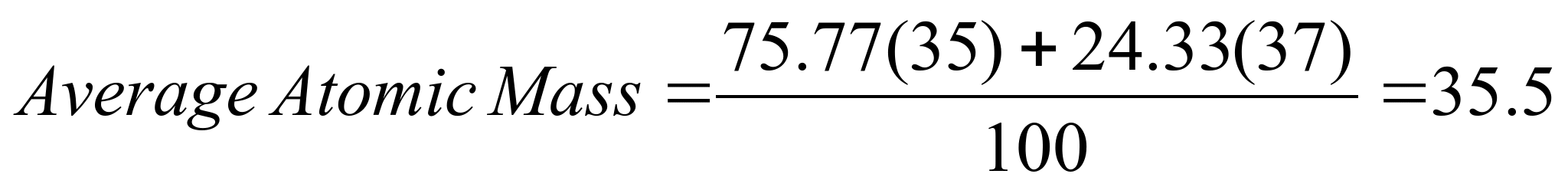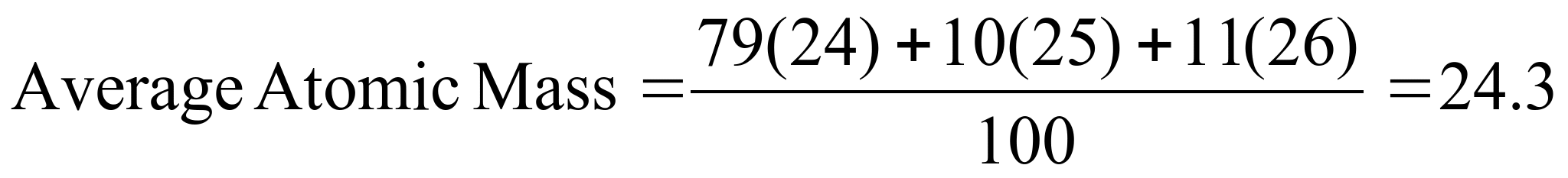Average
Atomic Mass
Look
at the picture below and answer the questions:

How many
particles are in the box?
What is the total
mass of the particles in the box?
What is the
average mass of a single particle in the box?
Answers
1. 8 + 6 = 14 particles
2. 8 particles with mass of 9 = 8*9 = 72
6 particles with mass of 7 = 6* 7 = 42
Total mass = 72+ 42 = 114
3. Average = Total mass/number of particles
Average mass = 114/14 = 8.1
Notice: none of the particles has this exact mass, but that is the
average mass of a particle across the whole sample.

7

7

7

7




7






7
How does this translate
into average atomic mass calculations? Recall that for each element
there may be different isotopes of the element that exist.
Isotope : atoms
of the same element that have different numbers of neutrons.
Recall, that if the
atoms are the same element, they must have the same number of
protons. However, if you change the number of neutrons in an atom,
you necessarily change the mass of that atom.
For example, one
isotope of chlorine is Cl-35 and one isotope is Chlorine-37.
Chlorine-35 has a mass of 35 amu, which comes from 17 protons and 18
neutrons. Chlorine-37 has a mass of 37 amu, which comes from 17
protons and 20 neutrons.
If you have a
measurable sample of chlorine, you will have a huge number of
chlorine atoms, some will be chlorine-35 and some will be
chlorine-37. However, in all likelihood, you will not be dealing
with this sample of particles one atom at a time, you will be dealing
with the whole group. You don’t want to measure the mass of each
particle individually, so you need to calculate the average atomic
mass.
However, we need some
information. We have the masses of the two types of particles but we
need to know how many of each kind there are. That is where data
about the natural abundance comes in handy.
Natural Abundance:
The number of atoms of a particular isotope that are found in
materials in nature. This number is frequently expressed as a
percent.
For example, for
Chlorine the natural abundances of the two isotopes are as follows:
Cl-35 : 75.78% and
Cl-37: 24.33% (Approximately a 3:1 ratio)
We could represent this
as follows:
1. Total number of
particles = 8
2. Total mass =
6(35) +2(37) = 284
3. Average mass =
284/8 = 35.5








But what if we had a
much larger sample, with millions of atoms. We wouldn’t want to
count out them out to do the calculation.
Instead, we can use the
percentages to count atoms. If we use percents as our way of
counting atoms, the average atomic mass formula becomes:
A verage
Atomic mass =
verage
Atomic mass =
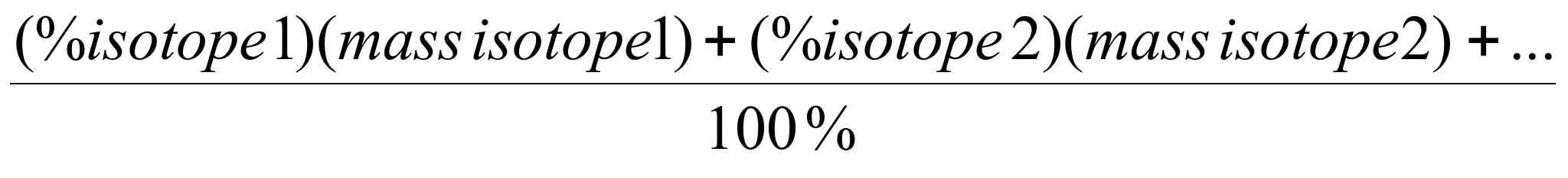

Notice: This is just
like the example above. The total mass is the percent of each
particle (our count of how many) * the mass of the particle.
Since we are counting
particles in percent, the total number of particles is 100%.
Example 1:
Determine the average atomic mass of chlorine using the above
formula.
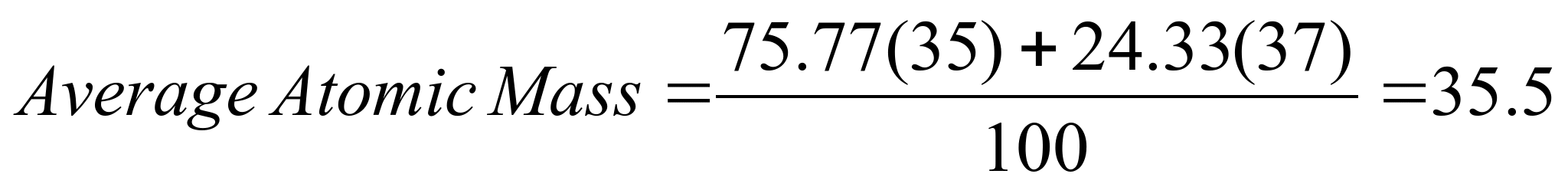
Notice, this is the
same number as we calculated above, and closely agrees with the
average mass for Cl found on the periodic table.
Example 2:
There are 3 naturally occurring isotopes of magnesium. The relative
abundance of each is shown in the table below. Using this
information, determine the average atomic mass.
-
|
Isotope
|
Mass
|
% Natural Abundance
|
|
Mg- 24
|
24
|
79
|
|
Mg-25
|
25
|
10
|
|
Mg-26
|
26
|
11
|
To find the average
atomic mass you would calculate:
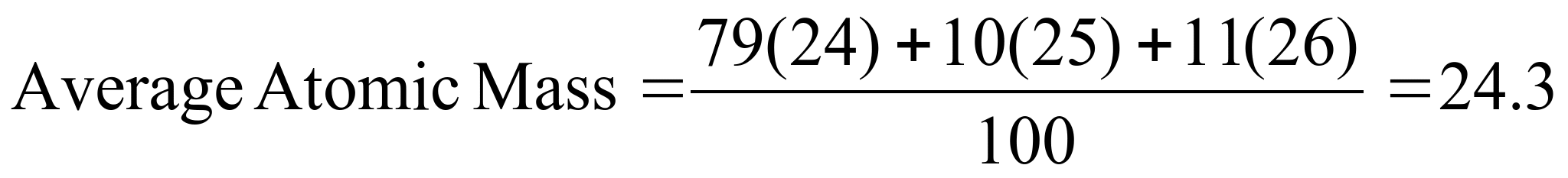
This is in agreement
with the average atomic mass for Mg seen on the periodic table.
Example 3:
Nickel (Ni) has an average atomic mass of 58.69. Use this
information to determine the naturally occurring isotopes of nickel.
Answer: You cannot do
this! There is no way to know what the naturally occurring elements
of nickel are except to take samples of nickel and test them (or look
at the results of a test someone else has completed). When you do
so, you find that there are 5 naturally occurring isotopes of nickel,
with the following relative abundances:
-
|
Isotope
|
Mass
|
% Natural Abundance
|
|
Ni-58
|
58
|
68.3
|
|
Ni-59
|
59
|
0
|
|
Ni-60
|
60
|
26.1
|
|
Ni-61
|
61
|
1.13
|
|
Ni-62
|
62
|
3.59
|
|
Ni-63
|
63
|
0
|
|
Ni-64
|
64
|
0.91
|
Again, we cannot fill
in the above table just based on the periodic table.
The most abundant
isotope is Ni-58. Nickel has an atomic number of 28, which means it
always has 28 protons. Therefore, Ni-58 must have 30 neutrons.






 verage
Atomic mass =
verage
Atomic mass =